

Thus, the theoretical yield of iron (Fe) in a reaction of 17.25 grams of 2Fe 2O 3 and 4.5 grams of 3C is 12.065 g. Let’s put it all together using the theoretical yield formula: The stoichiometry of Fe in the balanced equation above is 4.
#STOICHIOMETRY EQUATION MAKER FREE#
Feel free to use a molar mass calculator to calculate the molecular weight of your compound. The molecular weight of iron (Fe) is 55.845 g/mol. We found that 2Fe 2O 3 was the limiting reagent above, and we know there are 0.05401 moles. Let’s continue the example above and calculate the theoretical yield of iron (Fe) given a reaction of 17.25 grams of 2Fe 2O 3 and 4.5 grams of 3C. You’re now ready to use the formula above to calculate the theoretical yield. Step Four: Apply the Theoretical Yield Formula So, the moles of limiting reagent are equal to the mass of limiting reagent in grams divided by its molecular weight in g/mol, multiplied by the limiting reagent stoichiometry in the reaction, as found above when balancing the equation. Limiting reagent = mass / molecular weight × stoichiometry You can use the following formula to calculate this: Step Three: Find the Moles of Limiting ReagentĪfter you have found the limiting reagent, you’ll need to calculate how many moles of the limiting reagent will be in the reaction. Since there are 0.1249 moles of 3C available but only 0.0360067 moles will be reacted, there is an excess which means that 3C is the excess reactant and the limiting reactant is 2Fe 2O 3. The coefficients in front of each reactant is the reactant’s stoichiometry.Ĭontinuing the example above, if 17.25 grams of 2Fe 2O 3 are reacted with 4.5 grams of 3C, which reactant is the limiting reagent?Īssuming all of the 2Fe 2O 3 were reacted:Ġ.05401 moles × 2/3 = 0.0360067 moles of 3C reacted

Now, let’s add a coefficient to the hydrogen atoms on the left so that they equal the hydrogen atoms on the right.
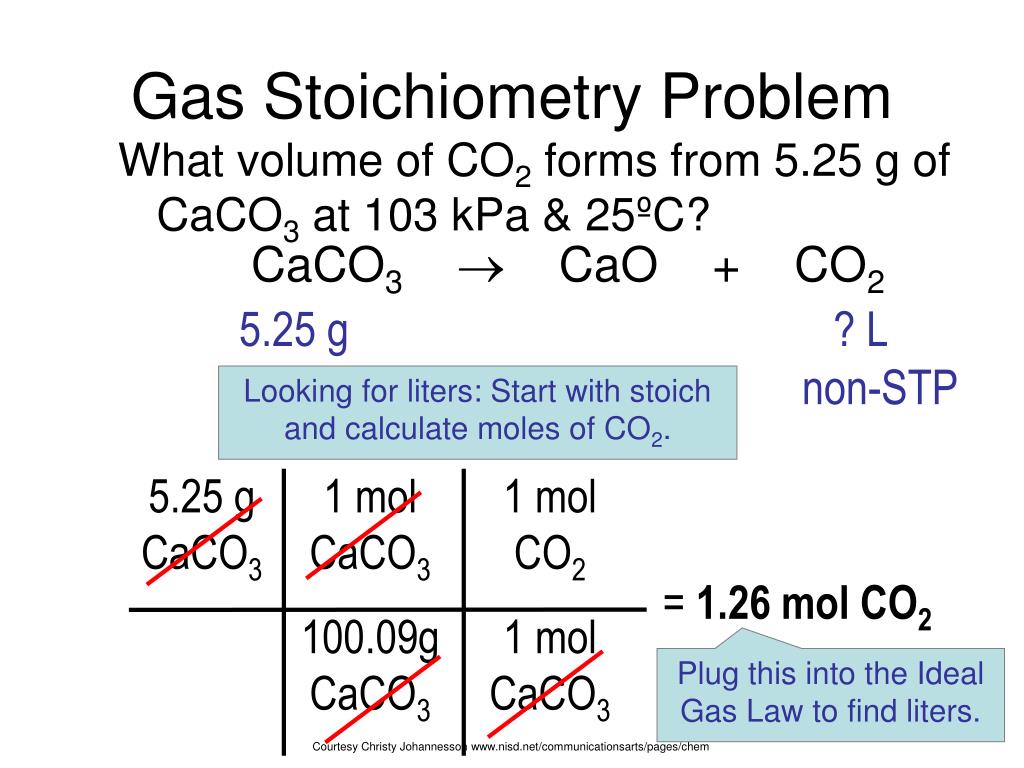
This equation still isn’t balanced as there are now two nitrogen atoms and six hydrogen atoms on the right, but on the left, there are two nitrogen atoms and two hydrogen atoms. Since there is one nitrogen atom on the right, add a coefficient of 2 before it to equal the amount of nitrogen atoms on the left. Let’s start with balancing the amount of nitrogen. To balance this equation, we’ll need to adjust the coefficients of some of the elements so that there are equal amounts of elements on each side of the equation. There are two atoms of nitrogen and two atoms of hydrogen on the left, but there is only one atom of nitrogen and three atoms of hydrogen on the right. The equation above is not balanced, however. In this example, nitrogen (N 2) and hydrogen (H 2) will react to create ammonia (NH 3). Let’s use an example of balancing the equation of N 2 + H 2 → NH 3. The goal is to create an equation for exactly the same number of atoms of reactants resulting in a desired product with the same number of atoms. The first step to using the theoretical yield equation is to create a balanced chemical equation.


 0 kommentar(er)
0 kommentar(er)
